Fenbendazole, a benzimidazole anthelmintic agent, treats parasitic infections in animals. Latest investigations recommend it might have antitumor properties, which has prompted its investigation as a potential disease therapy, including for pancreatic pancreatic cancer. Moreover, its safety and adequacy in humans are not deeply rooted. This article looks at the ongoing proof of how fenbendazole for humans is used to treat pancreatic cancer treatment and features the related dangers.
What is fenbendazole?

Fenbendazole treats parasitic worm infections in dogs and has been an anthelmintic in circulation since the early 1970s.
However, in recent years, numerous published and peer-reviewed papers and case reports have shown that fenbendazole is also very effective in treating aggressive human cancers. This is believed to be due to the following vital points that distinguish fenbendazole from other cancer-fighting drugs:
As mentioned above, a considerable number of scientific publications support and demonstrate its practical use in the treatment of some aggressive human cancers.
For example:
Fenbendazole alone has been reported to be an effective treatment for tumor regression in patients with diffuse large B-cell lymphoma metastatic cancer, renal cell carcinoma, and bladder cancer.
- It has few side effects and is very safe for humans to use.
- It is available without a prescription in many countries.
- It is highly cost-effective to produce.
How does Fenbendazole work?
Fenbendazole’s anti-cancer effects are very similar to those of the plant alkaloid group, including the cancer drug Taxol. In addition, due to its unique mode of action and exceptional safety profile, fenbendazole’s toxicity is far lower than that of conventional chemotherapies.
Based on numerous observations and findings, it has been shown that the origin of some types of cancer is related to and triggered by viruses, parasites, and the like.
It is even possible that this is the case in many more cases than we realize, mainly when the cancer cells are located in a “fertile ground” characterized by weakened immunity combined with specific genetic predispositions.
Therefore, it is considered that drugs should be used against parasites, worms, and lactate. Other diseases are also considered part of a comprehensive cancer treatment concept that may also include conventional cancer therapies.
Fenbendazole For Humans Overview
Fenbendazole is not a drug commonly used to treat humans like mebendazole. It is typically used in animals (including mammals, birds, and fish) with parasites and is known to kill numerous parasitic worms. Such as whipworms, hookworms, and some tapeworms.
Drug-Induced Liver Injury from Self-Administered Fenbendazole in NSCLC Patient
Fenbendazole, known by the brand names Panacur or Safe-Guard, was first discovered years ago through our research into cancer treatment. However, it has recently caught our attention again through the well-known story of a man who successfully treated his small cell lung cancer with the worm drug.
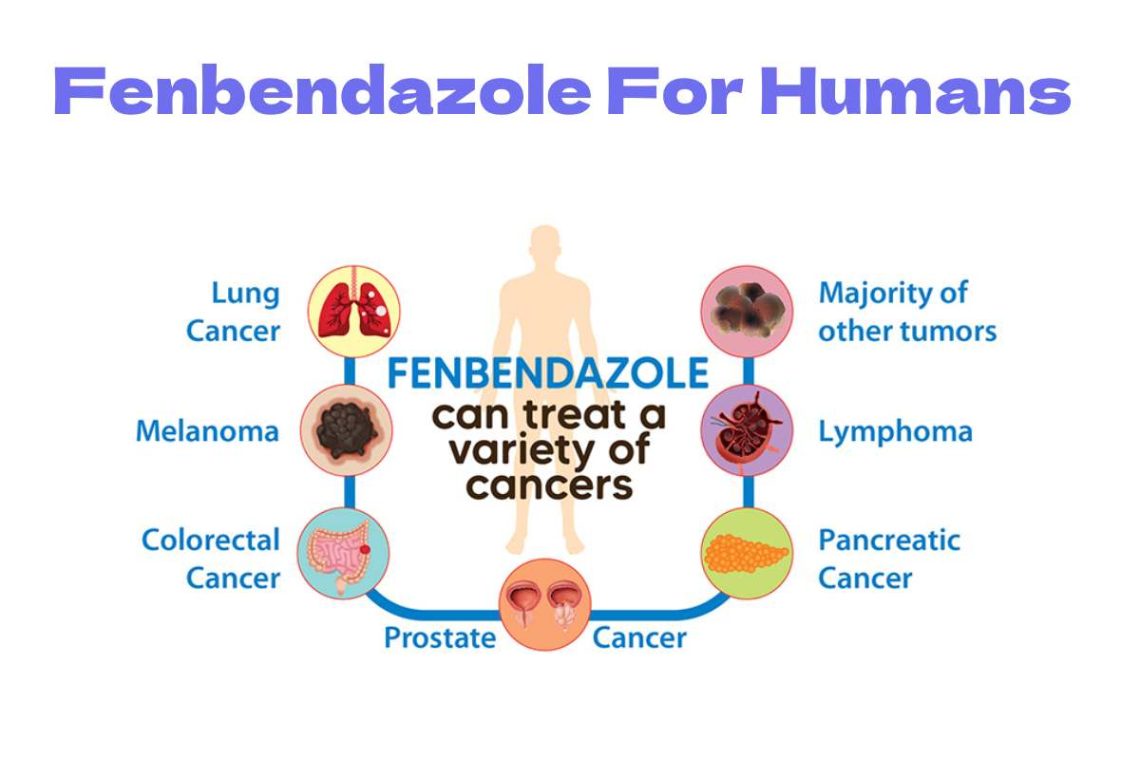
Since then, a dedicated website and Facebook group have served as a platform to share his own experiences and those of other patients who have experienced the benefits of using fenbendazole in treating cancers. Such as nonsmall cell lung cancer, melanoma, colon cancer, prostate cancer, stage four pancreatic cancer, and other types.
These recent reports are just further evidence of the existing and growing scientific evidence demonstrating the anti-cancer potential of numerous drugs in the benzimidazole class. Therefore, fenbendazole has significant anti-cancer potential in humans, as does mebendazole.
Even a few examinations have shown fenbendazole to be more viable than mebendazole. In one such review, fenbendazole was viewed as more potent than mebendazole and different medications in battling Cryptococcus neoformans, a pioneering growth found worldwide that can cause cryptococcal meningitis in specific individuals.
Various scientific articles have suggested that fenbendazole has anti-cancer properties. In this work, fenbendazole acts as a moderate microtubule destabilizer. It causes cancer cell death by modulating several cellular pathways.
Fenbendazole for Humans Anticancer Mechanisms and Potential
Recent investigations demonstrate that fenbendazole is a promising microtubule-interfering agent with antineoplastic activity. It disrupts multiple cellular pathways. It eliminates cancer cells by interfering with proteasomal and microtubule functions. Blocking glucose uptake by repressing GLUT4 development and possibly reactivating the tumor suppressor gene p53.
Fenbendazole’s exceptional activity on tubulin varies from vinca alkaloids. This difference permits it to supplement, rather than contend with, other cancer treatments. These include surgery, radiotherapy, and medications like sodium dichloroacetate (DCA) and berberine. It has shown effectiveness in combination with DCA, enhancing overall anticancer effects.
Fenbendazole for humans – is it safe for human consumption?
Although fenbendazole was initially commonly used to treat parasitic worms in animals. A report available from the European Medicines Agency states:
Fenbendazole appears to be well tolerated in humans following oral exposure:
- single oral dose up to 2,000 mg/person
- 500 mg/person for 10 consecutive days
However, long-term exposure has not yet been scientifically proven. This is probably due to the drug’s nature—the parasitic infections are usually eliminated in no more than 1 to 2 weeks.
Nevertheless, many people have been taking fenbendazole daily for years as a prophylactic to prevent cancer from recurring or as an active treatment against their tumors. The treatment appears to be safe and rarely, if ever, causes side effects.
Risks and Adverse Effects
The well-being profile of fenbendazole in people remains unclear. Anecdotal reports, for example, the case of an 80-year-old patient with nonsmall cell cellular breakdown in the lungs who experienced severe liver injury after self-directing fenbendazole, raise worries about its hepatotoxicity. The Naranjo Adverse Drug Reaction Probability Scale used in this case indicated a probable adverse reaction to fenbendazole. It highlights the requirement for alert and further examination.
Discussion
The likely utilization of fenbendazole in pancreatic cancer treatment is captivating; however, it requires thorough scientific approval. The absence of human clinical preliminaries implies that its pharmacokinetics, ideal dosing, and long-term security are obscure. Besides, the impact of social media in advancing unconfirmed medicines requires that medical services experts guide patients in getting dependable data and making informed choices.
Conclusion
Fenbendazole is potentially an anticancer specialist, especially in preclinical examinations for pancreatic disease. However, vigorous clinical proof of its use in humans isn’t yet available. The risks, including possible hepatotoxicity, must be carefully considered. Exhaustive clinical preliminaries are fundamental to establishing well-being and adequacy in people.